Welcome to “Off the Clock,” a little something that lands somewhere between Timeless & Timely.
I send out this fun look at language and words every other Saturday as bonus content. If someone sent this to you, please consider subscribing.

Rolling out of bed, you feel some aches and pains. Maybe you pulled a muscle and need a cream with some lidocaine. Perhaps your joints ache, causing you to reach for a bottle of acetaminophen or ibuprofen.
Whatever the causes of some health issues, we find solutions in chemical compounds with which the body interacts. We know them by their brand names and their generic names, but have you wondered how they got those names in the first place?
Potential drugs go through a rigorous safety review before approval — and so do their names. In order to avoid confusion with other similar drugs, according to Pfizer, “a single name for a drug candidate can take years to get approval from regulatory authorities, in an entirely separate process from the approval of the drug, itself.”1
Eliminating Confusion
When scientists discover that a potential drug that holds promise, the naming processes begins with the generic name. In 1961, the United States created an organization called the United States Adopted Names Council (USAN) to oversee the naming process.2
The FDA joined the USAN in 1967 and coordinates with the World Health Organization’s International Nonproprietary Names Programme (INN) to ensure the drug's generic name is globally consistent. This universal name allows individuals to access the medication by the same name, regardless of where they are in the world. This is particularly useful for travelers who may need to obtain their medication abroad.3
What’s in a Name
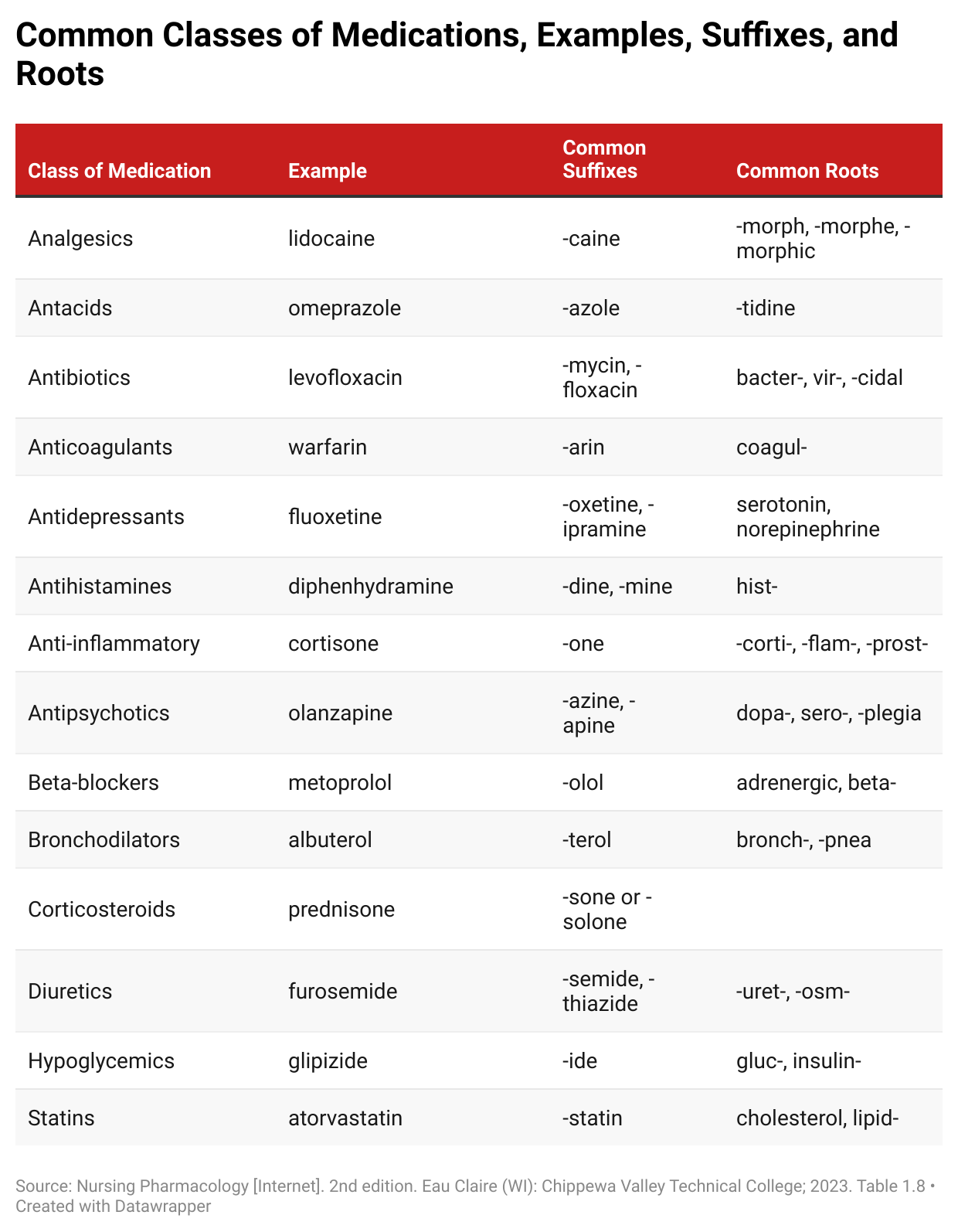
Generic drug names have two parts: a prefix and a suffix. The suffix acts as a scientific family name to describe the way the drug works in the body, while the prefix is often chosen to reflect the drug’s chemical structure or therapeutic class.
Generic Naming Requirements
A simple checklist outlines the process for selecting an appropriate name, guided by these necessities: